Triple Pressed Stearic Acid
(TPSA)
Stearic Acid
from Egypt to the world
From the heart of Egypt, and by Oleo Misr our stearic acid is rewriting the rules of success.
With an unwavering commitment to quality, sustainability, and innovation, we're not just rewriting the rules, we're creating a brand-new playbook for your business.
Explore the Possibilities 
Whether you're in cosmetics, pharmaceuticals, or any other industry, stearic acid is your versatile ally, promising enhanced product performance, optimized manufacturing, improved texture, and incredible cost-efficiency.
It's not just a product; it's a promise of excellence.
INCI Name
Stearic Acid
Chemical Formula
C18H36O2
CAS No.
57-11-4
Stearic Acid

1. Introduction
Stearic acid, also known as octadecanoic acid, is a saturated fatty acid with the molecular formula C18H36O2.
It is a waxy, white, and odorless solid that is derived from various vegetable fats and oils.
Ha as an important chemical compound with a wide array of applications in industries such as cosmetics, food, pharmaceuticals, and manufacturing.
2. Chemical Structure of Stearic Acid
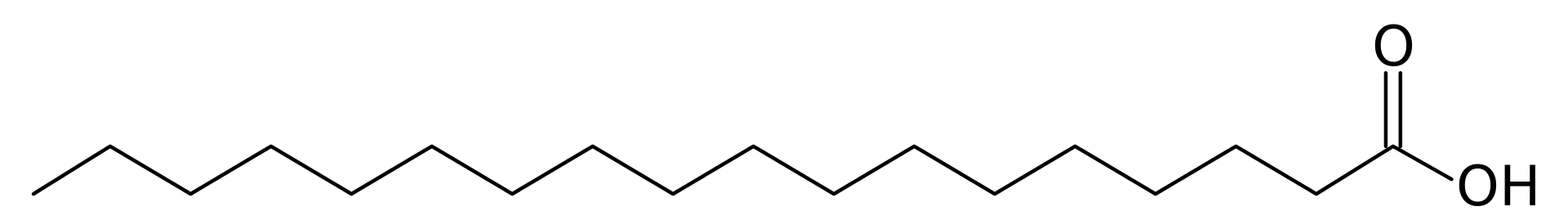
The chemical structure consists of a long hydrocarbon chain and a carboxyl group at the end.
Here is the structural formula: CH3(CH2)16CO2H.
3. Physical Properties
It exhibits several notable physical properties:
- Appearance: White, waxy solid
- Molecular Weight: 284.48 g/mol
- Melting Point: Approximately 69.6°C (157.3°F)
- Boiling Point: Approximately 383°C (721°F)
- Solubility: Insoluble in water; soluble in organic solvents such as ethanol and ether.
4. Chemical Properties
It is a versatile compound with various chemical properties:
- Acidic Nature: As a fatty acid, it can react with bases to form salts. These salts are commonly known as stearates.
- Hydrophobic: Stearic acid repels water, making it an effective water-resistant agent.
- Emulsifying Agent: It can act as an emulsifier, helping to mix water and oil-based substances.
- Reactivity: Stearic acid can undergo esterification and amination reactions to produce a wide range of derivatives for different applications.
5. Uses & Application
Stearic acid has a myriad of applications across various industries, some of which include:
- Cosmetics and Personal Care
It is a key ingredient in the cosmetic industry, used in products such as creams, lotions, and soaps due to its emulsifying and thickening properties. It provides stability and consistency to these products.
- Pharmaceuticals
In pharmaceuticals, stearic acid serves as a lubricant in tablet and capsule formulations, ensuring easy release from molds and preventing sticking.
- Food Industry
It is used as a food additive, primarily as a softening agent in chewing gum. It also helps in preventing sugar crystallization in candies.
- Candle Manufacturing
It is a crucial component in the production of candles, improving the hardness and texture of the wax.
- Rubber and Plastics
Acts as a processing aid and mold release agent in the manufacturing of rubber and plastic products.
- Textile Industry
It is utilized in the textile industry to impart water repellency and softness to fabrics.
- Industrial Lubricants
Stearic acid derivatives are used as additives in industrial lubricants to reduce friction and improve stability.
- Paper Industry
It is used as a sizing agent to improve the printability and water resistance of paper products.
- Construction Industry
Also, it is used as an additive in concrete and mortar to improve workability and reduce water penetration, enhancing the strength and durability of these materials.
- Adhesives
Considered a key component in hot melt adhesives, contributing to adhesive strength and flexibility.
- Metal Processing
In metalworking, stearic acid is used as a lubricant in the extrusion of aluminum and other metals, facilitating the shaping and forming processes.
- Automotive Industry
It is utilized in the manufacturing of rubber and plastic automotive components, ensuring durability and performance.
- Building and constructions
It is used in the production of construction materials, such as sealants and caulk, to enhance their adhesive properties.
These applications demonstrate the versatility of stearic acid and its ability to enhance product performance in various industries. Its unique properties make it a valuable ingredient with a wide range of uses.



Specifications
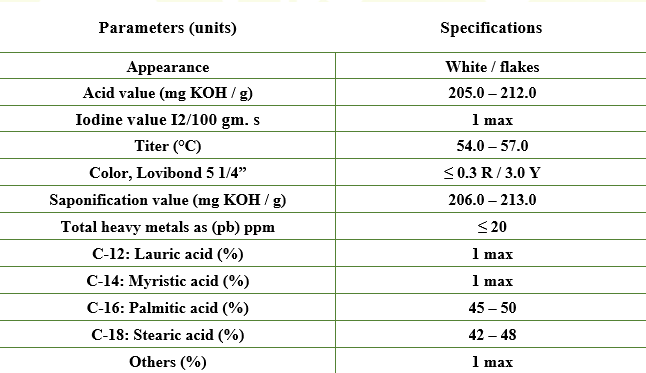
Stearic Acid Tripple Pressed (42:48-1)
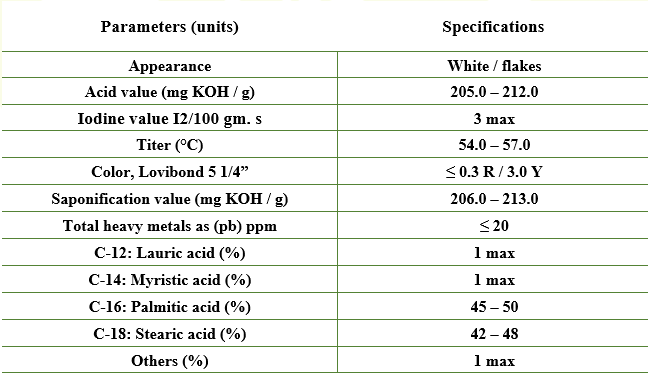
Stearic Acid Tripple Pressed (42:48-3)
 Partner with the Pioneers
Partner with the Pioneers 
At Oleo Misr, we're not just a manufacturer; we're collaborators in your success story.
Our expertise, custom solutions, global reach, and dedication to sustainability are the tools you need to reshape your industry.
Reach out to us today
to explore the endless opportunities.
Get in touch now and join the revolution of excellence!
Connect Now!
All Rights Reserved | Oleo Misr for Oleochemicals 2026


